The stereoselectivity of the Julia olefination is determined by the radical intermediate. The intermediates, both the cis and trans, can equilibrate allowing the. O. H. R2. R1. R2. +. Base. Overview of the Julia-Kocienski Olefination. Evans’ Group Literature Seminar. Scott Peterson. Sept. 26, Title 9/25/03 PM. The Julia-Lythgoe olefination involves the nucleophilic addition of lithiosulfones to carbonyl compounds, acylation, and reductive treatment of.

| Author: | Gale Malagrel |
| Country: | Eritrea |
| Language: | English (Spanish) |
| Genre: | Literature |
| Published (Last): | 19 March 2018 |
| Pages: | 424 |
| PDF File Size: | 11.21 Mb |
| ePub File Size: | 15.50 Mb |
| ISBN: | 387-9-70651-678-7 |
| Downloads: | 54193 |
| Price: | Free* [*Free Regsitration Required] |
| Uploader: | Tygole |
Search articles by author Paul R. Back to tab navigation.
A first possible mechanism proceeds through a planar radical that can rotate freely about the C-C bond. From Wikipedia, the free encyclopedia.
Julia olefination
The stereochemistry of the alkene 6 is independent of the stereochemistry of the sulfone intermediate 4. The article was received on 19 Aug and first published on 04 Nov If you are not the author of this article and you wish to reproduce material from it in a third party non-RSC publication you must formally request permission using Copyright Clearance Center.
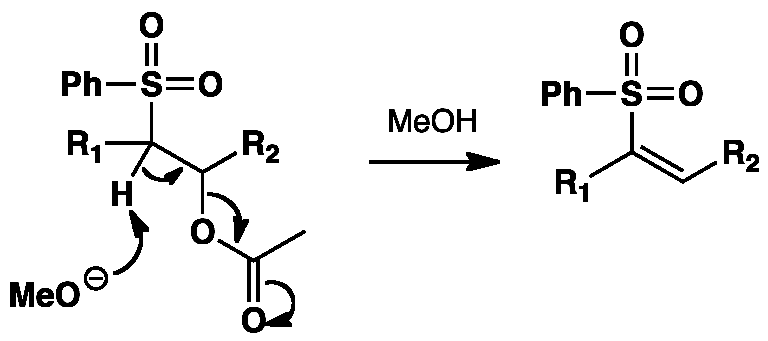
In other projects Wikimedia Commons. More recently, a modified version that avoids this step was developed. Application to the Synthesis of Maculalactone B N. Site Search any all words. Modified Julia Olefination on Anhydrides: The Jullia olefination also known as the Julia—Lythgoe olefination is the chemical reaction jupia in organic chemistry of phenyl sulfones 1 with aldehydes or ketones to give alkenes olefins 3 after alcohol functionalization and reductive elimination using sodium amalgam [1] [2] or SmI 2.
All four steps can be carried out in a single reaction vessel, and use of R 3 X is optional. If you are the author of this article you still need to obtain permission to reproduce kulia whole article in a third party publication with the exception of reproduction of the whole article in a thesis or dissertation. This page was last edited on 16 Augustat In addition, there are fewer problems with scale-up than with the classical variant.
The exact mechanism of the sodium amalgam reduction is unknown but has been shown to proceed oelfination a vinylic radical species 5 [11].
Julia-Lythgoe Olefination
In contrast to the classical Julia Olefination, the Modified Julia Olefination offers the possibility of saving one or two synthesis steps. The former version is sometimes referred to as oleifnation Julia-Lythgoe olefination, whereas the latter is called the Julia-Kocienski olefination.
Other hetero cyclic substituents can assume these roles, and offer somewhat different selectivity:. Site Search any all words Main Categories. The addition of a phenylsulfonyl carbanion to an aldehyde or ketone leads to an intermediate alcohol, which is esterified in situ. This may take some time to load. Whether the anti or syn intermediate is generated can be influenced to some extent by the choice of reaction conditions:.
Information about reproducing material from RSC articles with different licences is available on our Permission Requests page. The reductive elimination with sodium amalgam to furnish the alkene takes place in a second step. The following table shows the selectivity and yields for BT and PT sulfones in various solvents, where they olecination first metalated with various bases and then olefunation with an aldehyde.
In the reductive variant, the adduct is usually acylated and then treated with a reducing agent, such as sodium amalgam [6] [7] or SmI 2. The classical Julia Olefination with sodium amalgam might possibly proceed via an initial elimination to an alkenyl sulfone, which would then undergo homolytic cleavage involving single electron transfer.
The benzothiazolyl group BT can play several roles: The intermediates that form react further to give E – and Z -isomers of the alkene:.
Julia-Lythgoe Olefination | Chem-Station Int. Ed.
This transformation highly favors formation of the E -alkene. By using this site, you agree to the Terms of Use and Privacy Policy.

Keck demonstrated in that when the sodium amalgam reaction is run in MeOD as solvent, deuterium is incorporated into the product, in contrast to the absence of incorporation seen in the SmI 2 reduction. One adaptation of the Julia-Kocienski olefination gives the synthesis of the stilbenoid resveratrola natural compound found in common foods like grapes, wines and nuts.
Even though the carbanion is not configurationally or conformationally stable, it will prefer an arrangement with the R-groups further apart that will later lead to the E -alkene:. If the material has been adapted instead of reproduced from the original RSC publication “Reproduced from” can be substituted with “Adapted from”.