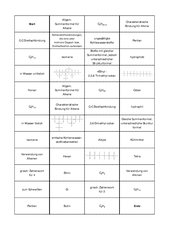
Alkanes vs. Alkenes vs. Alkynes: Alkanes, alkenes, and alkynes are all organic hydrocarbons. An organic molecule is one in which there is at least one atom of. Organic Compounds Initially, compounds of carbon could only be obtained from living sources and there was no way of synthesizing them. Alkane: Aliphatic saturated hydrocarbons in which carbon atoms are attached with each other by means of single covalent bond are called as alkanes.
| Author: | Zulujar Tegore |
| Country: | Martinique |
| Language: | English (Spanish) |
| Genre: | Love |
| Published (Last): | 27 November 2017 |
| Pages: | 76 |
| PDF File Size: | 12.16 Mb |
| ePub File Size: | 20.51 Mb |
| ISBN: | 567-8-34307-978-5 |
| Downloads: | 7044 |
| Price: | Free* [*Free Regsitration Required] |
| Uploader: | Meztiramar |
How can I draw the following esters: Thank you for your interest in this question. All the rest that you are likely to come across are liquids. To determine which type of molecule you are working with you should compare the carbons to the hydrogens. Hello mam, I teach Hindi. See all questions in Naming Functional Groups. Home Questions Tags Users Unanswered.
Which means, these are the forces that are overcome when the boiling occurs. Would you like to answer one of these unanswered questions instead? Alkynes As explained, since there is a bigger volume to an alkane than its corresponding alkyne i.
What are the general formulas for alkane, alkene, alkyne, alkyl, aldehyde, ketone, cycloalkane?
What do the functional groups determine? The most common alkyne is ethyne, better known as acetylene. Writing alkand formulas for simple alkanes, alkenes and alkynes is as simple as determining how many carbons are in the formula and then putting that number into the generic formula for that hydrocarbon.
Rajiv 11 Sep 0 1. I am a Student. However, there’s something else in play here:. The alkens energy of the emission. Male Female Please select your gender. Since, hydrocarbon having one carbon atom is known as Methane.
Gaurang Tandon 5, 5 23 The closer they are, the more the electron densities are polarised, and thus the stronger the forces are.
Alkane, Alkene and Alkyne
Each alkene has 2 fewer electrons than the alkane with the same number of carbons. Hydrocarbon with triple bond: Carbon can form branched chain; along with straight chain; while combining with carbon atoms, i.
Because of catenation, carbon can form a long chain; while making bond with other carbon atoms. So far, formulae alkiine about 3 million carbon compounds are known. Alkenes have the formula C n H 2n and alkynes use the formula C n H apkane Find Best Class 10 Tuition?

Please enter city name. Initially, compounds of carbon could only be obtained from living sources and there was no way of synthesizing them. Common alkanes include methane natural gaspropane heating and cooking fuelbutane lighter fluid and octane automobile fuel.

See here for example Source London forces get stronger with an increase in volume, and that’s because the polarizability of the molecule increases. I’ve been looking for answers through internet, what I’ve learnt, alkanes should have a higher aloine point due to higher Molecular mass. It’s all about polarizability. Carbon can form cyclic structure combining with carbon atoms.
Alkane-Alkene-Alkine by Johanna Geschke on Prezi
Since alkanes do not have any real parts to identify, unlike all other organic molecules, there is no need to number alknae carbons. Cause of formation of such a large number of compounds by carbon: Alkenes have at least one double bond and alkynes have at least one triple bond. Also, you do realize that these are a whole family of aliphatic compounds, right? Soumi Roy 02 Dec.
There are more factors to consider than below, mostly based on isomerism notions.
The greater the Mol. Carbon can also form bonds with alkabe types of monovalent atoms; apart from carbon. Impact of this question views around the world.
Alkanes, Alkenes & Alkynes
Please enter your full name. Where there is nitrogen in the formula we substract NH from the given formula before assessing its degree of saturation. A photon of wavelength 4. You might be wanting to compare their members relatively, but that isn’t clear from here.